Introducing PV-Pro: Powering Safety, Precision, and Compliance.
An innovative cornerstone in the global pharmacovigilance safety network, empowering advanced medicinal product vigilance management
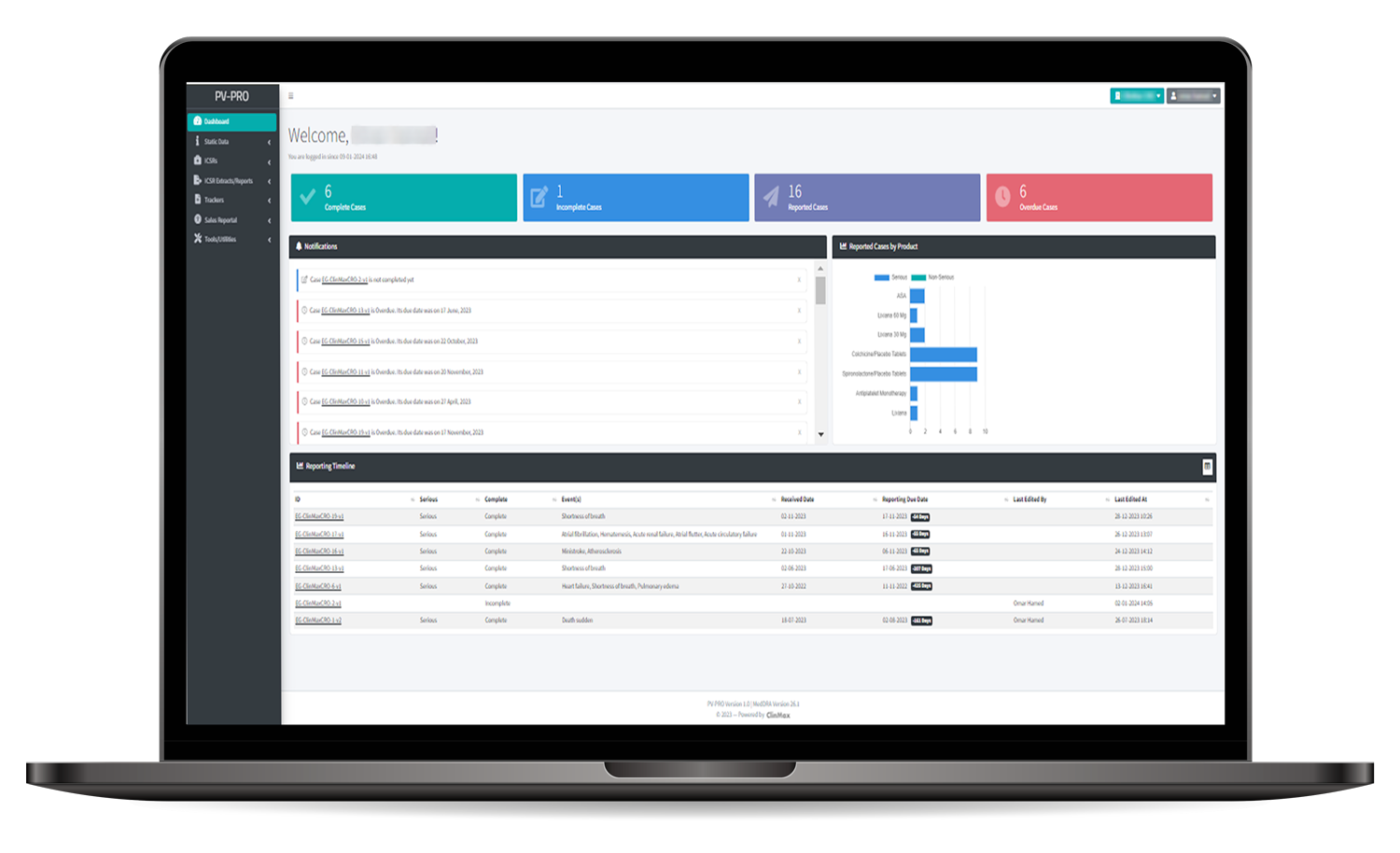

is a pioneering solution at the core of the global pharmacovigilance safety network. With a fully validated ICH E2B-R3 Database and integration with MedDRA, it facilitates comprehensive safety information processing. PV PRO offers a cost-effective solution with its user-friendly interface, streamlining the creation, review, submission, and maintenance of pharmacovigilance event reports alongside with other features supporting the Pharmacovigilance department.
Key Feature
- Multi-language input
- E2B R3 compliant XML report
- Configurable workflows
- Multi-tenant access
- Data export in Excel, Word, XML and PDF
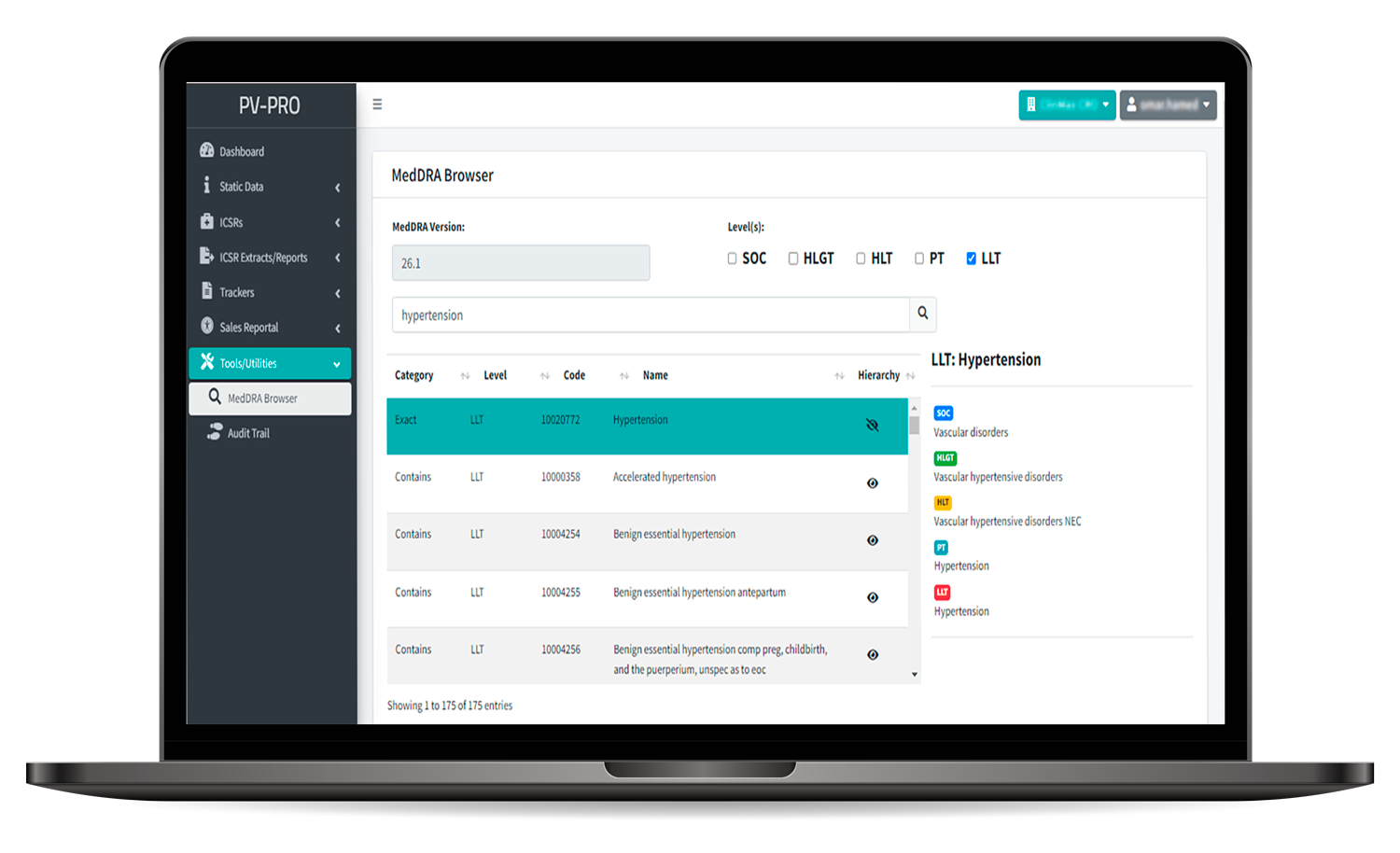
- In-built MedDRA coding tool with auto-coding option
- Comprehensive Audit Trail
- Cross-field validation to ensure E2B compliance
- Query chat for communication
- Manage multiple clients in one place
- Signal detection and management using qualitative and quantitative (statistical, machine learning, neural network) methods
- Duplicate search
- EDI Gateways with partners or competent authorities.
- Notifications / alerts on actions to be taken and upcoming deadlines.
Seamless Implementation
Comprehensive Data Security
Adaptable Solution
Efficient Safety Reporting and Data Integrity Assurance
PV PRO streamlines safety report submissions in XML format to regulatory authorities and external stakeholders. Boasting an intelligent case validator and audit trail, PV-Pro ensures data accuracy and integrity. Operating as a secure and centralized safety database, it excels in efficiently processing pharmacovigilance activities, enhanced by seamless MedDRA integration. This platform is designed to optimize operational efficiency, fostering a seamless and effective workflow for your business success.
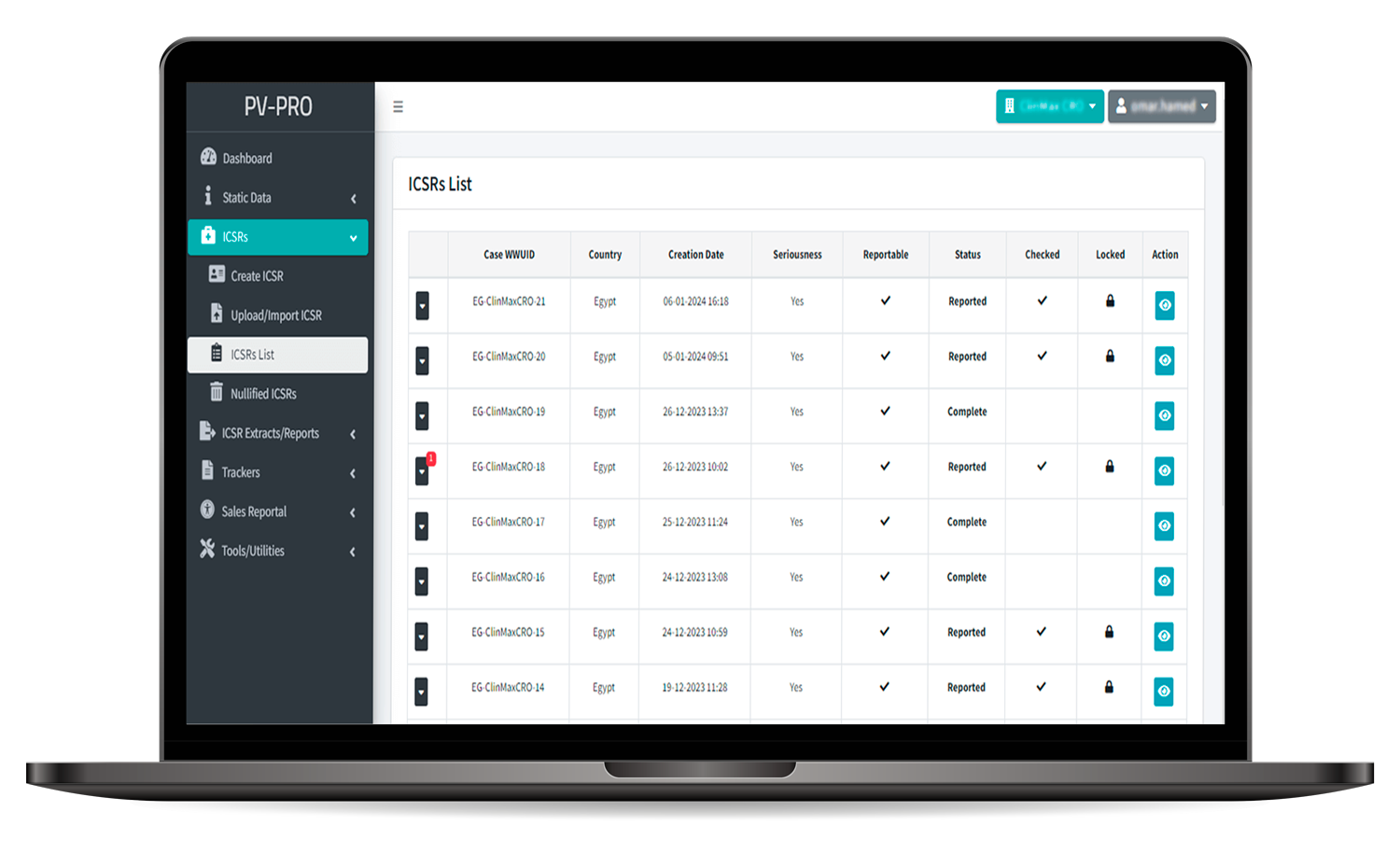
Adverse Event Reporting:
- Streamlined system for reporting and documenting adverse events related to medicinal products, supporting XML formats for reporting Individual Case Safety Reports (ICSRs).
- Ensures compliance with regulatory requirements and facilitates prompt reporting to relevant authorities.
Literature Surveillance:
- Ongoing monitoring of scientific literature and publications for emerging safety information.
- Facilitates literature search for continuous monitoring of scientific publications.
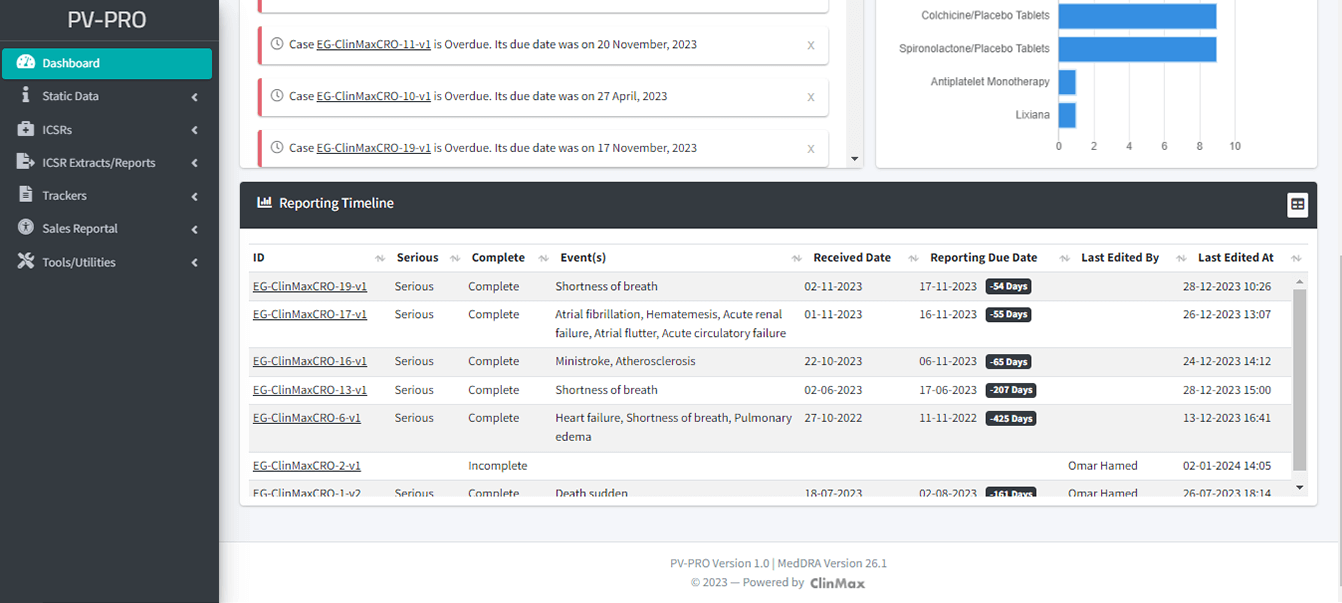
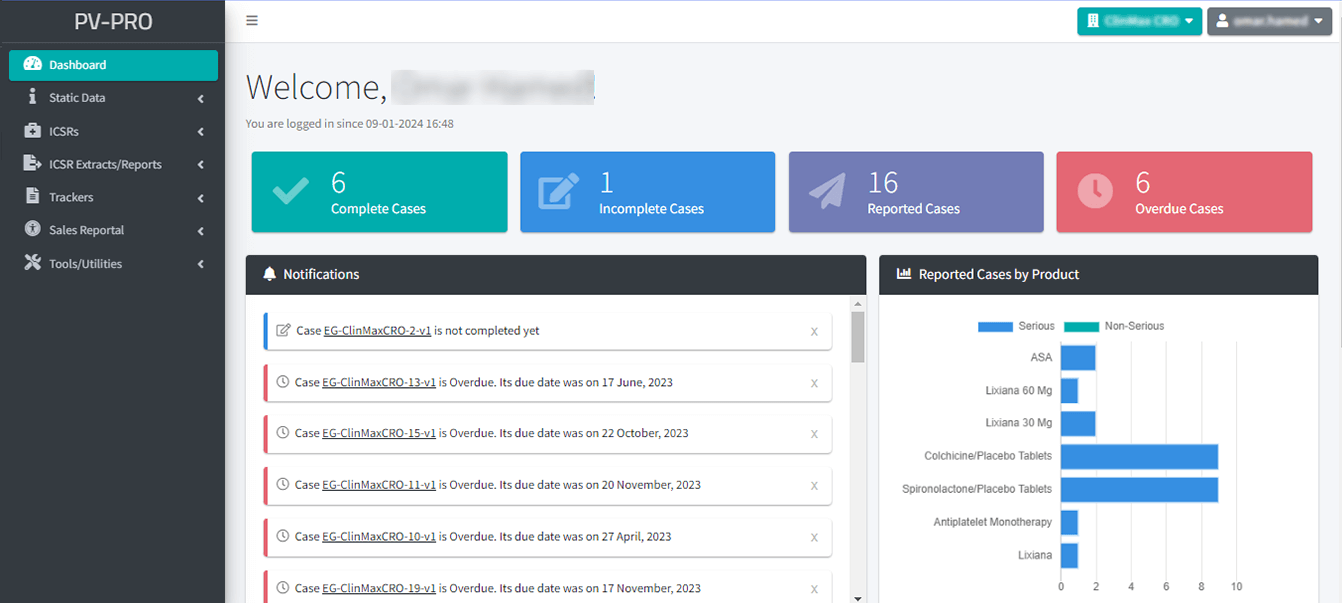
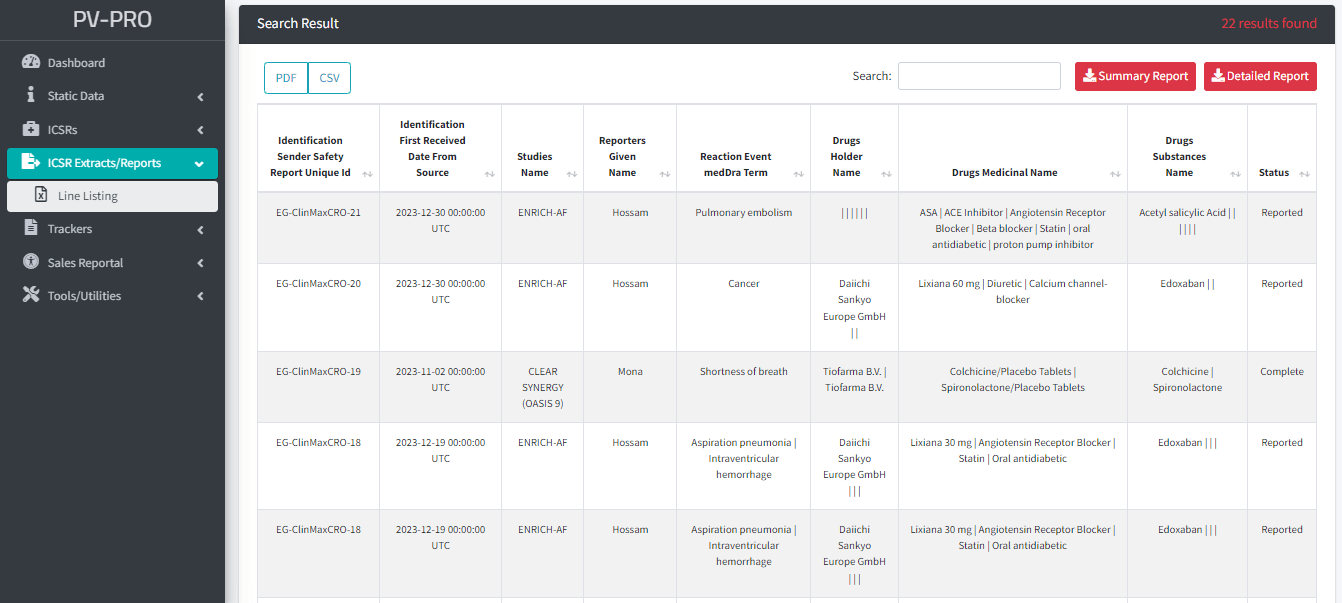
Reporting and Analytical Functions:
- Comprehensive tools for generating detailed reports on pharmacovigilance data.
- Advanced analytical functions to identify trends, patterns, and potential safety issues.
- Supports evidence-based decision-making for improved product safety and regulatory compliance.
Sales Representative / Patient Support Program Reporting Tool:
- Dedicated platform for efficient reporting by sales representatives and patient support programs.
- Enhances collaboration and ensures timely reporting of valuable information for a holistic approach to drug safety.
Our Coverage
- Europe
- Egypt
- Middle East
- Africa
our statistics
+3000
Users
+48
Countries
3Mn+
Cases
1134
Case studies
Have we picked your interest?
Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes.